Tap water: Why the water quality is so important for the functionality of the coolant
Lesezeit: 4 Min. | 09.10.2018
Content
Minimum requirements for tap water
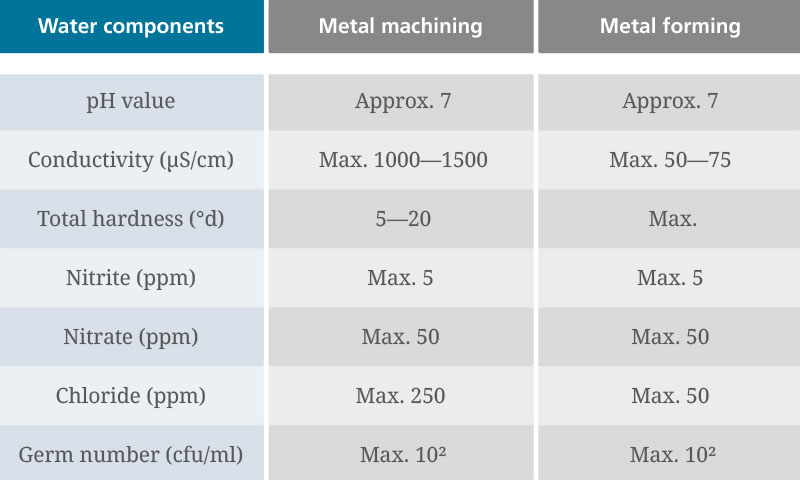
The pH value and total hardness are of particular importance. A pH value of 7 is optimal. In this case, the tap water will act as neutral. pH values of up to 9 are still permissible as long as there are no other reasons not to use this tap water.
Tap water with a pH value < 6 is unacceptable. Water with such a low pH could lower the pH value of the coolant emulsion when freshly applied. This results in reduced corrosion protection.
The pH value is not the only important factor for attaining optimal tap water. The total hardness is also important and is one of the most important application technology parameters.
If the water is too hard, this may cause the hardness components it contains to react with anionic emulsifiers to form poorly soluble compounds — known as lime soaps. This can result in clogged filters and deposits on workpieces and tools.
Tap water that is too weak also has adverse consequences for emulsions, as it promotes the formation of foam.
As a rule:
The optimum hardness range for tap water is around 5 to 20°d
If your tap water is very soft, we have a range of special products available to use. In the case of extremely low water
hardnesses of < 5°d, the addition of a harder mains water, if available, may help. It is possible to induce a change in the alkaline earth content of the tap water by adding a hardener containing Ca2+.
This type of manipulation is only required with fresh applications, as the total hardness of emulsions increases during use. Conversely, we recommend replenishing the volume losses with deionised water when using tap waters with a relatively high total hardness.